Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hirato, Misaki*; Yokoya, Akinari*; Baba, Yuji*; Mori, Seiji*; Fujii, Kentaro*; Wada, Shinichi*; Izumi, Yudai*; Haga, Yoshinori
Physical Chemistry Chemical Physics, 25(21), p.14836 - 14847, 2023/05
Times Cited Count:2 Percentile:77.93(Chemistry, Physical)Nakanishi, Takumi*; Hori, Yuta*; Shigeta, Yasuteru*; Sato, Hiroyasu*; Wu, S.-Q.*; Kiyanagi, Ryoji; Munakata, Koji*; Ohara, Takashi; Sato, Osamu*
Physical Chemistry Chemical Physics, 25(17), p.12394 - 12400, 2023/05
Times Cited Count:1 Percentile:56.86(Chemistry, Physical)Yamaguchi, Ko*; Kawaguchi, Daisuke*; Miyata, Noboru*; Miyazaki, Tsukasa*; Aoki, Hiroyuki; Yamamoto, Satoru*; Tanaka, Keiji*
Physical Chemistry Chemical Physics, 24(36), p.21578 - 21582, 2022/09
Times Cited Count:5 Percentile:70.33(Chemistry, Physical) path integral simulations
path integral simulationsThomsen, B.; Shiga, Motoyuki
Physical Chemistry Chemical Physics, 24(18), p.10851 - 10859, 2022/05
Times Cited Count:2 Percentile:16.81(Chemistry, Physical)Gao, D.*; Tang, X.*; Wang, X.*; Yang, X.*; Zhang, P.*; Che, G.*; Han, J.*; Hattori, Takanori; Wang, Y.*; Dong, X.*; et al.
Physical Chemistry Chemical Physics, 23(35), p.19503 - 19510, 2021/09
Times Cited Count:4 Percentile:36.54(Chemistry, Physical)Pressure-induced phase transition and polymerization of nitrogen-rich molecules are widely focused due to its extreme importance for the development of green high energy density materials. Here, we present a study of the phase transition and chemical reaction of 1H-tetrazole up to 100 GPa by using  Raman, IR, X-ray diffraction, neutron diffraction techniques and theoretical calculation. A phase transition above 2.6 GPa was identified and the high-pressure structure was determined with one molecule in a unit cell. The 1H-tetrazole polymerizes reversibly below 100 GPa, probably through a carbon-nitrogen bonding instead of nitrogen-nitrogen bonding. Our studies updated the structure model of the high pressure phase of 1H-tetrazole, and presented the possible intermolecular bonding route for the first time, which gives new insights to understand the phase transition and chemical reaction of nitrogen-rich compounds, and benefit for designing new high energy density materials.
Raman, IR, X-ray diffraction, neutron diffraction techniques and theoretical calculation. A phase transition above 2.6 GPa was identified and the high-pressure structure was determined with one molecule in a unit cell. The 1H-tetrazole polymerizes reversibly below 100 GPa, probably through a carbon-nitrogen bonding instead of nitrogen-nitrogen bonding. Our studies updated the structure model of the high pressure phase of 1H-tetrazole, and presented the possible intermolecular bonding route for the first time, which gives new insights to understand the phase transition and chemical reaction of nitrogen-rich compounds, and benefit for designing new high energy density materials.
Zhai, Y.*; Luo, P.*; Nagao, Michihiro*; Nakajima, Kenji; Kikuchi, Tatsuya*; Kawakita, Yukinobu; Kienzle, P. A.*; Z, Y.*; Faraone, A.*
Physical Chemistry Chemical Physics, 23(12), p.7220 - 7232, 2021/03
Times Cited Count:3 Percentile:27(Chemistry, Physical) -octylnitrilo-triacetamide (HONTA) complexes of americium and europium
-octylnitrilo-triacetamide (HONTA) complexes of americium and europiumToigawa, Tomohiro; Peterman, D. R.*; Meeker, D. S.*; Grimes, T. S.*; Zalupski, P. R.*; Mezyk, S. P.*; Cook, A. R.*; Yamashita, Shinichi*; Kumagai, Yuta; Matsumura, Tatsuro; et al.
Physical Chemistry Chemical Physics, 23(2), p.1343 - 1351, 2021/01
Times Cited Count:14 Percentile:81.5(Chemistry, Physical)The candidate An(III)/Ln(III) separation ligand hexa- -octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent (
-octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent ( -dodecane) radical cation of
-dodecane) radical cation of 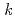 (HONTA + R
(HONTA + R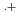 ) = (7.61
) = (7.61  0.82)
0.82)  10
10 M
M s
s obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed
obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed  -dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived (
-dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived ( 100 ns) HONTA radical cation as well as a longer-lived (
100 ns) HONTA radical cation as well as a longer-lived ( s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
Higuchi, Yuki*; Setoyama, Daigo*; Isegawa, Kazuhisa; Tsuchikawa, Yusuke; Matsumoto, Yoshihiro*; Parker, J. D.*; Shinohara, Takenao; Nagai, Yasutaka*
Physical Chemistry Chemical Physics, 23(2), p.1062 - 1071, 2021/01
Times Cited Count:6 Percentile:52.59(Chemistry, Physical)This study is the first report on liquid water and ice imaging conducted at a pulsed spallation neutron source facility. Neutron imaging can be utilised to visualise the water distribution inside polymer electrolyte fuel cells (PEFCs). Particularly, energy-resolved neutron imaging is a methodology capable of distinguishing between liquid water and ice, and is effective for investigating ice formation in PEFCs operating in a subfreezing environment. The distinction principle is based on the fact that the cross sections of liquid water and ice differ from each other at low neutron energies. In order to quantitatively observe transient freezing and thawing phenomena in a multiphase mixture (gas/liquid/solid) within real PEFCs with high spatial resolution, a pulsed neutron beam with both high intensity and wide energy range is most appropriate. In the validation study of the present work, we used water sealed in narrow capillary tubes to simulate the flow channels of a PEFC, and a pulsed neutron beam was applied to distinguish ice, liquid water and super-cooled water, and to clarify freezing and thawing phenomena of the water within the capillary tubes. Moreover, we have enabled the observation of liquid water/ice distributions in a large field of view (300 mm  300 mm) by manufacturing a sub-zero environment chamber that can be cooled down to -30
300 mm) by manufacturing a sub-zero environment chamber that can be cooled down to -30 C, as a step towards
C, as a step towards  visualisation of full-size fuel cells.
visualisation of full-size fuel cells.
Akutsu, Kazuhiro*; Cagnes, M.*; Tamura, Kazuhisa; Kanaya, Toshiji*; Darwish, T. A.*
Physical Chemistry Chemical Physics, 21(32), p.17512 - 17516, 2019/08
Times Cited Count:11 Percentile:53.26(Chemistry, Physical)We combined the deuterium labeling and neutron reflectivity techniques to determine the fine structure of the electric double layer structure in an imidazolium ionic liquid (IL). For this, a simple and large scale deuteration method for imidazolium ILs was developed, where the deuteration level can be systematically controlled.
Kusaka, Ryoji; Watanabe, Masayuki
Physical Chemistry Chemical Physics, 20(47), p.29588 - 29590, 2018/12
Times Cited Count:18 Percentile:70.02(Chemistry, Physical)Mechanistic understanding of solvent extraction of uranyl ions (UO
 ) by tributyl phosphate (TBP) will help improve the technology for the treatment and disposal of spent nuclear fuels. So far, it has been believed that uranyl ions in the aqueous phase are adsorbed to a TBP-enriched organic/aqueous interface, form complexes with TBP at the interface, and are extracted into the organic phase. Here we show that uranyl-TBP complex formation does not take place at the interface using vibrational sum frequency generation (VSFG) spectroscopy and propose an alternative extraction mechanism that uranyl nitrate, UO
) by tributyl phosphate (TBP) will help improve the technology for the treatment and disposal of spent nuclear fuels. So far, it has been believed that uranyl ions in the aqueous phase are adsorbed to a TBP-enriched organic/aqueous interface, form complexes with TBP at the interface, and are extracted into the organic phase. Here we show that uranyl-TBP complex formation does not take place at the interface using vibrational sum frequency generation (VSFG) spectroscopy and propose an alternative extraction mechanism that uranyl nitrate, UO (NO
(NO )
) , passes through the interface and forms the uranyl-TBP complex, UO
, passes through the interface and forms the uranyl-TBP complex, UO (NO
(NO )
) (TBP)
(TBP) , in the organic phase.
, in the organic phase.
Suzuki, Kento*; Miyazaki, Takaaki*; Takayanagi, Toshiyuki*; Shiga, Motoyuki
Physical Chemistry Chemical Physics, 20(41), p.26489 - 26499, 2018/11
Times Cited Count:1 Percentile:3.34(Chemistry, Physical)The direct photoionization of pure helium clusters and its subsequent short-time process have been studied by path integral molecular dynamics (PIMD) and ring-polymer molecular dynamics (RPMD) simulations. The PIMD simulations reproduced the experimental ionization spectra with a broad and asymmetric nature, which can be ascribed to the inhomogeneity of the energy levels of He atoms. From the RPMD simulations, it is found that the ionized helium cluster in the highly excited state brings about fast electronic state relaxation via nonadiabatic charge transfer and subsequently slow structural relaxation.
Ishii, Yusuke*; Komatsu, Kazuki*; Nakano, Satoshi*; Machida, Shinichi*; Hattori, Takanori; Sano, Asami; Kagi, Hiroyuki*
Physical Chemistry Chemical Physics, 20(24), p.16650 - 16656, 2018/06
Times Cited Count:4 Percentile:17.83(Chemistry, Physical)The structure of an aluminum layered hydroxide, boehmite ( -AlOOH), as a function of pressure was studied by using
-AlOOH), as a function of pressure was studied by using  synchrotron X-ray and neutron diffraction. Peak broadening and subsequent splitting, which are only found for hkl (h
synchrotron X-ray and neutron diffraction. Peak broadening and subsequent splitting, which are only found for hkl (h 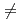 0) peaks in the X-ray diffraction patterns above 25 GPa, are explained by stacking disorder accompanied with a continuously increasing displacement of the AlO
0) peaks in the X-ray diffraction patterns above 25 GPa, are explained by stacking disorder accompanied with a continuously increasing displacement of the AlO octahedral layer along a-axis. This finding could be the first experimental result for the pressure-induced stacking disorder driven by the continuous layer displacement. The magnitude of the layer displacement was estimated from the X-ray scattering profile calculation based on the stacking disordered structure model. Hydrogen bond geometries of boehmite, obtained by structure refinements on the observed neutron diffraction patterns for deuterated sample up to 10 GPa, show linearly approaching O-D covalent and D
octahedral layer along a-axis. This finding could be the first experimental result for the pressure-induced stacking disorder driven by the continuous layer displacement. The magnitude of the layer displacement was estimated from the X-ray scattering profile calculation based on the stacking disordered structure model. Hydrogen bond geometries of boehmite, obtained by structure refinements on the observed neutron diffraction patterns for deuterated sample up to 10 GPa, show linearly approaching O-D covalent and D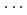 O hydrogen bond distances and they could merge below 26 GPa. The pressure-induced stacking disorder would make the electrostatic potential of hydrogen bonds asymmetric, yielding less chance for the proton-tunnelling.
O hydrogen bond distances and they could merge below 26 GPa. The pressure-induced stacking disorder would make the electrostatic potential of hydrogen bonds asymmetric, yielding less chance for the proton-tunnelling.
 O
O induced by swift heavy ions
induced by swift heavy ionsYoshioka, Satoru*; Tsuruta, Konosuke*; Yamamoto, Tomokazu*; Yasuda, Kazuhiro*; Matsumura, Sho*; Ishikawa, Norito; Kobayashi, Eiichi*
Physical Chemistry Chemical Physics, 20(7), p.4962 - 4969, 2018/02
Times Cited Count:5 Percentile:23.21(Chemistry, Physical)Cationic disorder in the MgAl O
O spinel induced by swift heavy ions was investigated using the X-ray absorption near edge structure. With changes in the irradiation fluences of 200 MeV Xe ions, the Mg K-edge and Al K-edge spectra were synchronously changed. The calculated spectra based on density function theory indicate that the change in the experimental spectra was due to cationic disorder between Mg in tetrahedral sites and Al in octahedral sites. These results suggest a high inversion degree to an extent that the completely random configuration is achieved in MgAl
spinel induced by swift heavy ions was investigated using the X-ray absorption near edge structure. With changes in the irradiation fluences of 200 MeV Xe ions, the Mg K-edge and Al K-edge spectra were synchronously changed. The calculated spectra based on density function theory indicate that the change in the experimental spectra was due to cationic disorder between Mg in tetrahedral sites and Al in octahedral sites. These results suggest a high inversion degree to an extent that the completely random configuration is achieved in MgAl O
O induced by the high density electronic excitation under swift heavy ion irradiation.
induced by the high density electronic excitation under swift heavy ion irradiation.
Kai, Takeshi; Yokoya, Akinari*; Ukai, Masatoshi*; Fujii, Kentaro*; Toigawa, Tomohiro; Watanabe, Ritsuko*
Physical Chemistry Chemical Physics, 20(4), p.2838 - 2844, 2018/01
Times Cited Count:22 Percentile:75.15(Chemistry, Physical)It is thought that complex DNA damage which induces in radiation biological effects is formed at radiation track end. Thus, the earliest stage of water radiolysis at the electron track end was studied to predict DNA damage. These results indicate that DNA damage sites comprising multiple nucleobase lesions with a single strand breaks can therefore be formed by multiple collisions of the electrons within three base pairs (3bp) of a DNA strand. This multiple damage site cannot be processed by base excision repair enzymes. However, pre-hydrated electrons can also be produced resulting in an additional base lesion more than 3bp away from the multi-damage site. This clustered damage site may be finally converted into a double strand break (DSB) when base excision enzymes process the additional base lesions. These DSBs include another base lesion(s) at their termini that escape from the base excision process and which may result in biological effects such as mutation in surviving cells.
Kusaka, Ryoji; Watanabe, Masayuki
Physical Chemistry Chemical Physics, 20(4), p.2809 - 2813, 2018/01
Times Cited Count:14 Percentile:60.51(Chemistry, Physical)Solvent extraction plays an integral part in the separation and purification of metals. Because extractants generally used as complexing agents for metal extractions, such as di-(2-ethylhexyl)phosphoric acid (HDEHP) for lanthanide extractions, are amphiphilic, they come to the organic/water interface, and the interface plays a crucial role as the site of the formation of metal complexes and subsequent transfer reaction to an organic phase. Despite the importance of the interface for solvent extractions, however, molecular-level structure of the interface is unclear because of experimental difficulty. Here we studied structure of a trivalent europium (Eu ) complex with HDEHP formed at HDEHP monolayer/water interface by heterodyne-detected vibrational sum frequency generation (HD-VSFG) spectroscopy. The study on the HDEHP/water interface enables us to investigate the structure of the interfacial Eu
) complex with HDEHP formed at HDEHP monolayer/water interface by heterodyne-detected vibrational sum frequency generation (HD-VSFG) spectroscopy. The study on the HDEHP/water interface enables us to investigate the structure of the interfacial Eu complex by excluding the migration of Eu
complex by excluding the migration of Eu into an organic phase after the complex formation at the interface. The interface-selective vibrational Im
into an organic phase after the complex formation at the interface. The interface-selective vibrational Im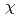
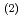 spectra observed by HD-VSFG of HDEHP/Eu(NO
spectra observed by HD-VSFG of HDEHP/Eu(NO )
) aqueous solution interface in the 2800-3500 cm
aqueous solution interface in the 2800-3500 cm region indicate that Eu
region indicate that Eu at the HDEHP/water interface is bonded by HDEHP from the air side and by water molecules from the water side. To the best of our knowledge, such metal complex structures have not been identified in the organic or water solutions.
at the HDEHP/water interface is bonded by HDEHP from the air side and by water molecules from the water side. To the best of our knowledge, such metal complex structures have not been identified in the organic or water solutions.
Sassi, M.*; Okumura, Masahiko; Machida, Masahiko; Rosso, K. M.*
Physical Chemistry Chemical Physics, 19(39), p.27007 - 27014, 2017/10
Times Cited Count:4 Percentile:17(Chemistry, Physical)no abstracts in English
Seki, Yusuke*; Takayanagi, Toshiyuki*; Shiga, Motoyuki
Physical Chemistry Chemical Physics, 19(21), p.13798 - 13806, 2017/06
Times Cited Count:8 Percentile:35.36(Chemistry, Physical)Ring-polymer molecular dynamics (RPMD) simulations have been performed to understand the photoexcitation dynamics of the Ag atom embedded in a low-temperature cluster consisting of 500 helium atoms, after the electronic excitation of the Ag atom. Along the RPMD trajectory the time evolution of the electronic wavefunction within the spin-orbit  P manifold is calculated, whereby the time-dependent Schr
P manifold is calculated, whereby the time-dependent Schr dinger equation and the RPMD equation of motion are coupled,
dinger equation and the RPMD equation of motion are coupled, 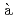 la Ehrenfest mean field approach. It is found from the simulations that the Ag atom is mostly ejected from the helium cluster with the average time of 100 ps after photoexcitation. The average velocity of the ejected Ag atom is estimated to be 60-70 m/s. These results are qualitatively in line with previous experimental findings.
la Ehrenfest mean field approach. It is found from the simulations that the Ag atom is mostly ejected from the helium cluster with the average time of 100 ps after photoexcitation. The average velocity of the ejected Ag atom is estimated to be 60-70 m/s. These results are qualitatively in line with previous experimental findings.
Hiyama, Miyabi*; Shiga, Motoyuki; Koga, Nobuaki*; Sugino, Osamu*; Akiyama, Hidefumi*; Noguchi, Yoshifumi*
Physical Chemistry Chemical Physics, 19(15), p.10028 - 10035, 2017/04
Times Cited Count:7 Percentile:31.12(Chemistry, Physical)In this study, the effect of hydration on the absorption spectra of oxyluciferin anion isomers in an aqueous solution is investigated for elucidating the influence of characteristic hydration structures. Using a canonical ensemble of hydration structures obtained from first-principles molecular dynamics simulations, the instantaneous absorption spectra of keto-, enol-, and enolate-type aqueous oxyluciferin anions at room temperature are computed from a collection of QM/MM calculations using an explicit solvent. It is demonstrated that the calculations reproduce experimental results concerning spectral shifts and broadening, for which traditional methods based on quantum chemistry and the Franck-Condon approximation fail because of the molecular vibrations of oxyluciferin anions and dynamical fluctuations of their hydration structures.
Ruiz-Barragan, S.*; Ribas Ari o, J.*; Shiga, Motoyuki
o, J.*; Shiga, Motoyuki
Physical Chemistry Chemical Physics, 18(47), p.32438 - 32447, 2016/12
Times Cited Count:8 Percentile:32.26(Chemistry, Physical)The use of high-temperature liquid water (HTW) as a reaction medium is a very promising technology in the field of green chemistry. In order to fully exploit this technology, it is crucial to unravel the reaction mechanisms of the processes carried out in HTW. In this work, the reaction mechanism of 2,5-hexanediol dehydration in HTW has been studied by means of three different ab initio simulations: string method, metadynamics and molecular dynamics in real time. It is found that the whole reaction involving the protonation, bond exchange and the deprotonation occurs in a single step without a stable intermediate. The hydrogen bonded network of surrounding water has a vital role in assisting an efficient proton relay at the beginning and at the end of the reaction. It is confirmed that the reaction is energetically most favorable in the SN2 pathway with an estimated barrier of 36 kcal/mol, which explains the high stereo selectivity and the reaction rate observed in experiment.
 (110)-(1
(110)-(1 2) surface using total-reflection high-energy positron diffraction (TRHEPD)
2) surface using total-reflection high-energy positron diffraction (TRHEPD)Mochizuki, Izumi*; Ariga, Hiroko*; Fukaya, Yuki; Wada, Ken*; Maekawa, Masaki; Kawasuso, Atsuo; Shidara, Tetsuo*; Asakura, Kiyotaka*; Hyodo, Toshio*
Physical Chemistry Chemical Physics, 18(10), p.7085 - 7092, 2016/03
Times Cited Count:19 Percentile:62.79(Chemistry, Physical)no abstracts in English